https://doi.org/10.1140/epjp/s13360-025-06486-8
Regular Article
Optimal control and sensitivity analysis of a mathematical model for MDR-TB transmission with advanced treatment strategies
1
Department of Mathematics, University of Malakand, Chakdara, Dir(L), 18800, Kpk, Pakistan
2
Air Conditioning Engineering Department, University of Warith Al-Anbiyaa, Karbala, Iraq
3
Department of Mechanical Engineering, Prince Mohammad Bin Fahd University, Al-Khobar, Saudi Arabia
4
Department of Chemistry and Its Teaching Methods, Tashkent State Pedagogical University, Tashkent, Uzbekistan
5
Department of Physics-Chemistry, University of Potsdam, Potsdam, Germany
6
Department of Physics, Northern Border University, Arar, Saudi Arabia
a
sislam@pmu.edu.sa
b
grasool@pmu.edu.sa
Received:
22
April
2025
Accepted:
26
May
2025
Published online:
19
June
2025
Tuberculosis (TB) remains a formidable global health challenge, exacerbated by the rise of drug-resistant strains like MDR-TB and XDR-TB. This study introduces an advanced eight-compartment deterministic model that transcends conventional frameworks by incorporating critical stages often overlooked in TB dynamics: chronic carriers, persistent cases, and advanced treatment pathways for drug-resistant TB, alongside vaccinated and recovered populations. The model’s novelty lies in its integration of time-dependent vaccination efficacy and adaptive treatment strategies, revealing that targeted vaccination campaigns outperform mere treatment expansion in reducing prevalence, a finding validated through real-world vulnerability indices. By deriving the basic reproduction number  , we establish threshold criteria for disease persistence and prove global stability using Lyapunov theory. Sensitivity analysis identifies transmission rate (
, we establish threshold criteria for disease persistence and prove global stability using Lyapunov theory. Sensitivity analysis identifies transmission rate (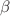 ) and vaccination coverage (
) and vaccination coverage ( ) as the most influential parameters, offering policymakers actionable levers for intervention. Our optimal control analysis demonstrates that combining time-varying strategies enhanced vaccination (
) as the most influential parameters, offering policymakers actionable levers for intervention. Our optimal control analysis demonstrates that combining time-varying strategies enhanced vaccination (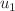 ), accelerated treatment (
), accelerated treatment ( ), and transmission suppression (
), and transmission suppression (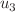 ) can reduce TB incidence by up to 40% in high-burden settings while optimizing resource allocation. Numerical simulations underscore the efficacy of these strategies, showing delayed peaks in infectious compartments and faster recovery rates under controlled scenarios. The model’s practical utility extends to designing cost-effective TB programs in resource-limited regions, particularly where socio-economic barriers impede treatment adherence. Future work will explore spatial heterogeneity and co-infections (e.g., HIV-TB) to further refine intervention scalability.
) can reduce TB incidence by up to 40% in high-burden settings while optimizing resource allocation. Numerical simulations underscore the efficacy of these strategies, showing delayed peaks in infectious compartments and faster recovery rates under controlled scenarios. The model’s practical utility extends to designing cost-effective TB programs in resource-limited regions, particularly where socio-economic barriers impede treatment adherence. Future work will explore spatial heterogeneity and co-infections (e.g., HIV-TB) to further refine intervention scalability.
Copyright comment Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
© The Author(s), under exclusive licence to Società Italiana di Fisica and Springer-Verlag GmbH Germany, part of Springer Nature 2025
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.




